Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
In which of the following are the formula of the ionic compound and the charge
on the metal ion shown correctly?
|
|
|
2.
|
Which of the following correctly represents an ion pair and the ionic compound
the ions form?
|
|
|
3.
|
In which of the following is the name and formula given correctly?
a. | sodium oxide, NaO | c. | cobaltous chloride, CoCl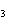 | b. | barium nitride, BaN | d. | stannic fluoride, SnF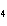 |
|
|
|
4.
|
Which of the following compounds contains the lead(II) ion?
|
|
|
5.
|
Which set of chemical name and chemical formula for the same compound is
correct?
a. | iron(II) oxide, Fe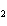 O O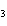 | c. | tin(IV) bromide, SnBr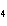 | b. | aluminum fluorate, AlF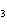 | d. | potassium
chloride, K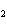 Cl Cl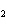 |
|
|
|
6.
|
What is the correct formula for potassium sulfite?
|
|
|
7.
|
What type of compound is CuSO 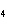 ? a. | monotomic ionic | c. | polyatomic ionic | b. | polyatomic covalent | d. | binary
molecular |
|
|
|
8.
|
Which polyatomic ion forms a neutral compound when combined with a group 1A
monatomic ion in a 1:1 ratio?
a. | ammonium | c. | nitrate | b. | carbonate | d. | phosphate |
|
|
|
9.
|
Sulfur hexafluoride is an example of a ____.
a. | monatomic ion | c. | binary compound | b. | polyatomic ion | d. | polyatomic
compound |
|
|
|
10.
|
Molecular compounds are usually ____.
a. | composed of two or more transition elements | b. | composed of positive
and negative ions | c. | composed of two or more nonmetallic
elements | d. | exceptions to the law of definite proportions |
|
|
|
11.
|
What is the ending for the names of all binary compounds, both ionic and
molecular?
|
|
|
12.
|
Binary molecular compounds are made of two ____.
a. | metallic elements | c. | polyatomic ions | b. | nonmetallic elements | d. | cations |
|
|
|
13.
|
In naming a binary molecular compound, the number of atoms of each element
present in the molecule is indicated by ____.
a. | Roman numerals | c. | prefixes | b. | superscripts | d. | suffixes |
|
|
|
14.
|
Which of the following correctly shows a prefix used in naming binary molecular
compounds with its corresponding number?
a. | deca-, 7 | c. | hexa-, 8 | b. | nona-, 9 | d. | octa-, 4 |
|
|
|
15.
|
Which of the following is a binary molecular compound?
a. | BeHCO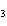 | c. | AgI | b. | PCl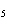 | d. | MgS |
|
|
|
16.
|
Which of the following formulas represents a molecular compound?
a. | ZnO | c. | SO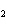 | b. | Xe | d. | BeF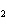 |
|
|
|
17.
|
When dissolved in water, acids produce ____.
a. | negative ions | c. | hydrogen ions | b. | polyatomic ions | d. | oxide ions |
|
|
|
18.
|
When naming acids, the prefix hydro- is used when the name of the acid
anion ends in ____.
|
|
|
19.
|
Which of the following shows both the correct formula and correct name of an
acid?
a. | HClO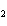 , chloric acid , chloric acid | c. | H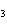 PO PO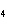 , phosphoric acid , phosphoric acid | b. | HNO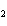 , hydronitrous
acid , hydronitrous
acid | d. | HI, iodic
acid |
|
|
|
20.
|
What is the name of H 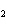 SO 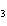 ? a. | hyposulfuric acid | c. | sulfuric acid | b. | hydrosulfuric acid | d. | sulfurous acid |
|
|
|
21.
|
When the name of an anion that is part of an acid ends in -ite, the acid
name includes the suffix ____.
|
|
|
22.
|
What is the formula for sulfurous acid?
|
|
|
23.
|
What is the formula for phosphoric acid?
|
|
|
24.
|
What is the formula for hydrosulfuric acid?
|
|
|
25.
|
Which of the following are produced when a base is dissolved in water?
a. | hydronium ions | c. | hydrogen ions | b. | hydroxide ions | d. | ammonium ions |
|
|
|
26.
|
How are bases named?
a. | like monatomic elements | c. | like ionic
compounds | b. | like polyatomic ions | d. | like molecular compounds |
|
|
|
27.
|
In any chemical compound, the elements are always combined in the same
proportion by ____.
a. | charge | c. | volume | b. | mass | d. | density |
|
|
|
28.
|
Select the correct formula for sulfur hexafluoride.
|
|
|
29.
|
What is the correct name for the compound CoCl 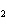 ? a. | cobalt(I) chlorate | c. | cobalt(II) chlorate | b. | cobalt(I) chloride | d. | cobalt(II)
chloride |
|
|
|
30.
|
What is the correct formula for barium chlorate?
a. | Ba(ClO)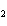 | c. | Ba(ClO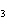 ) )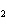 | b. | Ba(ClO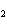 ) )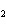 | d. | BaCl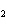 |
|