Matching
|
|
|
Match each item with the correct statement below. a. | monatomic ion | f. | cation | b. | acid | g. | binary compound | c. | base | h. | anion | d. | law of definite proportions | i. | polyatomic ion | e. | law of multiple
proportions |
|
|
|
1.
|
consists of a single atom with a positive or negative charge
|
|
|
2.
|
atom or group of atoms having a negative charge
|
|
|
3.
|
atom or group of atoms having a positive charge
|
|
|
4.
|
tightly-bound group of atoms that behaves as a unit and carries a net
charge
|
|
|
5.
|
compound composed of two different elements
|
|
|
6.
|
produces a hydrogen ion when dissolved in water
|
|
|
7.
|
produces a hydroxide ion when dissolved in water
|
|
|
8.
|
In any chemical compound, the masses of elements are always in the same
proportion by mass.
|
|
|
9.
|
when two elements form more than one compound, the masses of one element that
combine with the same mass of the other element are in the ratio of small, whole numbers
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
10.
|
What type of ions have names ending in -ide?
a. | only cations | c. | only metal ions | b. | only anions | d. | only gaseous
ions |
|
|
|
11.
|
When Group 2A elements form ions, they ____.
a. | lose two protons | c. | lose two electrons | b. | gain two protons | d. | gain two
electrons |
|
|
|
12.
|
What is the correct name for the N 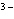 ion? a. | nitrate ion | c. | nitride ion | b. | nitrogen ion | d. | nitrite ion |
|
|
|
13.
|
When naming a transition metal ion that can have more than one common ionic
charge, the numerical value of the charge is indicated by a ____.
a. | prefix | c. | Roman numeral following the name | b. | suffix | d. | superscript after the name |
|
|
|
14.
|
Aluminum is a group 3A metal. Which ion does A1 typically form?
|
|
|
15.
|
In which of the following are the symbol and name for the ion given
correctly?
|
|
|
16.
|
Which of the following correctly provides the name of the element, the symbol
for the ion, and the name of the ion?
a. | fluorine, F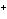 , fluoride ion , fluoride ion | c. | copper, Cu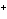 ,
cuprous ion ,
cuprous ion | b. | zinc, Zn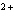 , zincate ion , zincate ion | d. | sulfur, S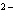 , sulfurous
ion , sulfurous
ion |
|
|
|
17.
|
The nonmetals in Groups 6A and 7A ____.
a. | lose electrons when they form ions | b. | have a numerical charge that is found by
subtracting 8 from the group number | c. | all have ions with a –1
charge | d. | end in -ate |
|
|
|
18.
|
What determines that an element is a metal?
a. | the magnitude of its charge | c. | when it is a Group A
element | b. | the molecules that it forms | d. | its position in the periodic table |
|
|
|
19.
|
What is the Stock name for chromic ion?
a. | chromium(I) ion | c. | chromium(III) ion | b. | chromium(II) ion | d. | chromium(IV)
ion |
|
|
|
20.
|
Which of the following is NOT a cation?
a. | iron(III) ion | c. | Ca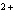 | b. | sulfate | d. | mercurous ion |
|
|
|
21.
|
In which of the following are the symbol and name for the ion given
correctly?
|
|
|
22.
|
Which of the following correctly provides the names and formulas of polyatomic
ions?
|
|
|
23.
|
An -ate or -ite at the end of a compound name usually indicates
that the compound contains ____.
a. | fewer electrons than protons | c. | only two
elements | b. | neutral molecules | d. | a polyatomic anion |
|
|
|
24.
|
Why are systematic names preferred over common names?
a. | Common names do not provide information about the chemical composition of the
compound. | b. | Common names are derived from the method used to obtain the
compound. | c. | Common names were assigned by the scientist who discovered the
compound. | d. | Common names are not very descriptive. |
|
|
|
25.
|
Which of the following compounds contains the Mn 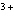 ion?
|
|
|
26.
|
How are chemical formulas of binary ionic compounds generally written?
a. | cation on left, anion on right | b. | anion on left, cation on
right | c. | Roman numeral first, then anion, then cation | d. | subscripts first,
then ions |
|
|
|
27.
|
Which of the following is true about the composition of ionic compounds?
a. | They are composed of anions and cations. | b. | They are composed of
anions only. | c. | They are composed of cations only. | d. | They are formed from two or more nonmetallic
elements. |
|
|
|
28.
|
Which of the following formulas represents an ionic compound?
|
|
|
29.
|
Which element, when combined with fluorine, would most likely form an ionic
compound?
a. | lithium | c. | phosphorus | b. | carbon | d. | chlorine |
|
|
|
30.
|
Which of the following shows correctly an ion pair and the ionic compound the
two ions form?
|