Matching
|
|
|
Match each item with the correct statement below. a. | electronegativity | f. | periodic law | b. | ionization energy | g. | cation | c. | atomic
radius | h. | period | d. | metal | i. | group | e. | transition
metal | j. | electrons |
|
|
|
1.
|
horizontal row in the periodic table
|
|
|
2.
|
vertical column in the periodic table
|
|
|
3.
|
A repetition of properties occurs when elements are arranged in order of
increasing atomic number.
|
|
|
4.
|
type of element that is a good conductor of heat and electric current
|
|
|
5.
|
type of element characterized by the presence of electrons in the d
orbital
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
6.
|
What is another name for the representative elements?
a. | Group A elements | c. | Group C elements | b. | Group B elements | d. | transition
elements |
|
|
|
7.
|
What is another name for the transition metals?
a. | noble gases | c. | Group B elements | b. | Group A elements | d. | Group C
elements |
|
|
|
8.
|
Which of the following elements is in the same period as phosphorus?
a. | carbon | c. | nitrogen | b. | magnesium | d. | oxygen |
|
|
|
9.
|
Each period in the periodic table corresponds to ____.
a. | a principal energy level | c. | an orbital | b. | an energy
sublevel | d. | a
suborbital |
|
|
|
10.
|
The modern periodic table is arranged in order of increasing atomic ____.
a. | mass | c. | number | b. | charge | d. | radius |
|
|
|
11.
|
Who arranged the elements according to atomic mass and used the arrangement to
predict the properties of missing elements?
a. | Henry Moseley | c. | John Dalton | b. | Antoine Lavoisier | d. | Dmitri
Mendeleev |
|
|
|
12.
|
Which of the following categories includes the majority of the elements?
a. | metalloids | c. | metals | b. | liquids | d. | nonmetals |
|
|
|
13.
|
Of the elements Pt, V, Li, and Kr, which is a nonmetal?
|
|
|
14.
|
To what category of elements does an element belong if it is a poor conductor of
electricity?
a. | transition elements | c. | nonmetals | b. | metalloids | d. | metals |
|
|
|
15.
|
In which of the following sets is the symbol of the element, the number of
protons, and the number of electrons given correctly?
a. | In, 49 protons, 49 electrons | c. | Cs, 55 protons, 132.9
electrons | b. | Zn, 30 protons, 60 electrons | d. | F, 19 protons, 19
electrons |
|
|
|
16.
|
The atomic number of an element is the total number of which particles in the
nucleus?
a. | neutrons | c. | electrons | b. | protons | d. | protons and
electrons |
|
|
|
17.
|
What element has the electron configuration 1 s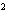 2 s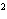 2 p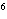 3 s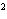 3 p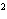 ? a. | nitrogen | c. | silicon | b. | selenium | d. | silver |
|
|
|
18.
|
Which of the following is true about the electron configurations of the noble
gases?
a. | The highest occupied s and p sublevels are completely
filled. | b. | The highest occupied s and p sublevels are partially
filled. | c. | The electrons with the highest energy are in a d sublevel. | d. | The electrons with
the highest energy are in an f sublevel. |
|
|
|
19.
|
Elements that are characterized by the filling of p orbitals are
classified as ____.
a. | groups 3A through 8A | c. | inner transition metals | b. | transition
metals | d. | groups 1A and
2A |
|
|
|
20.
|
Which of the following electron configurations is most likely to result in an
element that is relatively inactive?
a. | a half-filled energy sublevel | b. | a filled energy sublevel | c. | one empty and one
filled energy sublevel | d. | a filled highest occupied principal energy
level |
|
|
|
21.
|
Which subatomic particle plays the greatest part in determining the properties
of an element?
a. | proton | c. | neutron | b. | electron | d. | none of the
above |
|
|
|
22.
|
Which of the following elements is a transition metal?
a. | cesium | c. | tellurium | b. | copper | d. | tin |
|
|
|
23.
|
Which of the following groupings contains only representative elements?
a. | Cu, Co, Cd | c. | Al, Mg, Li | b. | Ni, Fe, Zn | d. | Hg, Cr, Ag |
|
|
|
24.
|
Which of the following is true about the electron configurations of the
representative elements?
a. | The highest occupied s and p sublevels are completely
filled. | b. | The highest occupied s and p sublevels are partially
filled. | c. | The electrons with the highest energy are in a d sublevel. | d. | The electrons with
the highest energy are in an f sublevel. |
|
|
|
25.
|
What are the Group 1A and Group 7A elements examples of?
a. | representative elements | c. | noble gases | b. | transition
elements | d. | nonmetallic
elements |
|
|
|
26.
|
Of the elements Fe, Hg, U, and Te, which is a representative element?
|
|
|
27.
|
How does atomic radius change from top to bottom in a group in the periodic
table?
a. | It tends to decrease. | c. | It first increases, then decreases. | b. | It tends to
increase. | d. | It first
decreases, then increases. |
|
|
|
28.
|
How does atomic radius change from left to right across a period in the periodic
table?
a. | It tends to decrease. | c. | It first increases, then decreases. | b. | It tends to
increase. | d. | It first
decreases, then increases. |
|
|
|
29.
|
What causes the shielding effect to remain constant across a period?
a. | Electrons are added to the same principal energy level. | b. | Electrons are added
to different principal energy levels. | c. | The charge on the nucleus is constant.
| d. | The atomic radius increases. |
|
|
|
30.
|
Atomic size generally ____.
a. | increases as you move from left to right across a period | b. | decreases as you
move from top to bottom within a group | c. | remains constant within a
period | d. | decreases as you move from left to right across a
period |
|