Matching
|
|
|
Match each item with the correct statement below. a. | absolute zero | e. | mass | b. | Kelvin temperature scale | f. | significant figure | c. | Celsius temperature
scale | g. | precision | d. | weight | h. | accuracy |
|
|
|
1.
|
the lowest point on the Kelvin scale
|
|
|
2.
|
the SI scale for temperature
|
|
|
3.
|
the force of gravity on an object
|
|
|
4.
|
the non-SI scale for temperature
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
5.
|
Which of the following volumes is the smallest?
a. | one microliter | c. | one milliliter | b. | one liter | d. | one deciliter |
|
|
|
6.
|
What is the SI unit of mass?
a. | liter | c. | candela | b. | joule | d. | kilogram |
|
|
|
7.
|
What is the temperature of absolute zero measured in 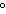 C? a. | –373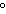 C C | c. | –173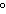 C C | b. | –273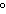 C C | d. | –73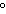 C C |
|
|
|
8.
|
Which temperature scale has no negative temperatures?
a. | Celsius | c. | Joule | b. | Fahrenheit | d. | Kelvin |
|
|
|
9.
|
What is the boiling point of water in kelvins?
a. | 0 K | c. | 273 K | b. | 100 K | d. | 373 K |
|
|
|
10.
|
Which of the following mass units is the largest?
|
|
|
11.
|
The weight of an object ____.
a. | is the same as its mass | c. | is not affected by
gravity | b. | depends on its location | d. | is always the same |
|
|
|
12.
|
What is the temperature –34 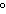 C expressed in kelvins? a. | 139 K | c. | 239 K | b. | 207 K | d. | 339 K |
|
|
|
13.
|
If the temperature changes by 100 K, by how much does it change in 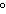 C?
|
|
|
14.
|
Chlorine boils at 239 K. What is the boiling point of chlorine expressed in
degrees Celsius?
|
|
|
|
|
|
15.
|
What is the quantity 0.0075 meters expressed in centimeters? Use the table above
to help you.
a. | 0.075 cm | c. | 7.5 cm | b. | 0.75 cm | d. | 70.5 cm |
|
|
|
16.
|
A train travels at a speed of 30 miles per hour. If 1 mile = 1.6 kilometers, how
fast is the train traveling in kilometers per minute?
a. | 0.4 km/min | c. | 0.8 km/min | b. | 0.6 km/min | d. | 1.0 km/min |
|
|
|
17.
|
The density of mercury is 5,427 kg/(m3). If the density of water is
1.0 g/mL, will mercury float or sink in water?
a. | Mercury will float because the density of mercury is 0.005427 g/mL, which is less
than the 1.0 g/mL density of water. | b. | Mercury will float because the density of
mercury is 0.05427 g/mL, which is less than the 1.0 g/mL density of water. | c. | Mercury will sink
because the density of mercury is 5.427 g/mL, which is greater than the 1.0 g/mL density of
water. | d. | Mercury will sink because the density of mercury is 5,427 g/mL, which is greater than
the 1.0 g/mL density of water. |
|
|
|
18.
|
Which of the following equalities is NOT correct? Use the table above to help
you.
a. | 100 cg = 1 g | c. | 1 cm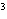 = 1 mL = 1 mL | b. | 1000 mm = 1
m | d. | 10 kg = 1
g |
|
|
|
19.
|
A cubic meter is about the same as the volume occupied by a ____.
a. | kilogram of water | c. | washing machine | b. | cup of milk | d. | basketball
arena |
|
|
|
20.
|
The quantity 44 liters expressed in cubic meters is ____.
a. | 0.000 044 m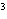 | c. | 0.44 m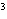 | b. | 440 000 m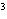 | d. | 0.044
m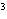 |
|
|
|
21.
|
The quantity 67 liters expressed in cubic meters is ____.
a. | 0.067 m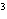 | c. | 0.67 m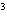 | b. | 0.000 067 m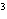 | d. | 670 000
m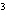 |
|
|
|
22.
|
The quantity 80 liters expressed in cubic meters is ____.
a. | 0.000 080 m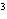 | c. | 0.080 m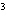 | b. | 0.80 m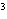 | d. | 800 000
m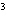 |
|
|
|
23.
|
The quantity 34 liters expressed in cubic meters is ____.
a. | 0.34 m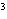 | c. | 340 000 m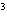 | b. | 0.034 m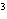 | d. | 0.000 034
m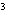 |
|
|
|
24.
|
Density is found by dividing ____.
a. | mass by volume | c. | mass by area | b. | volume by mass | d. | area by mass |
|
|
|
25.
|
What is the density of an object having a mass of 8.0 g and a volume of 25
cm 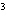 ? a. | 0.32 g/cm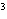 | c. | 3.1 g/cm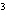 | b. | 2.0 g/cm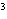 | d. | 200
g/cm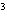 |
|
|
|
26.
|
What is the volume of 80.0 g of ether if the density of ether is 0.70
g/mL?
|
|
|
27.
|
What is the volume of 45.6 g of silver if the density of silver is 10.5
g/mL?
a. | 0.23 mL | c. | 479 mL | b. | 4.34 mL | d. | none of the
above |
|
|
|
28.
|
If a liter of water is heated from 20 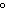 C to 50 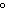 C, what happens to its
volume? a. | The volume decreases. | b. | The volume increases. | c. | The volume first
increases, then decreases. | d. | The volume first decreases, then
increases. |
|
|
|
29.
|
If the temperature of a piece of steel decreases, what happens to its
density?
a. | The density decreases. | b. | The density increases. | c. | The density does not
change. | d. | The density first increases, then decreases. |
|
|
|
30.
|
As the density of a substance increases, the volume of a given mass of that
substance ____.
a. | increases | c. | decreases | b. | is not affected | d. | fluctuates |
|