Matching
|
|
|
Match each item with the correct statement below. a. | absolute zero | e. | mass | b. | Kelvin temperature scale | f. | significant figure | c. | Celsius temperature
scale | g. | precision | d. | weight | h. | accuracy |
|
|
|
1.
|
closeness to true value
|
|
|
2.
|
narrowness of range of measurements
|
|
|
3.
|
known or estimated in a measurement
|
|
|
4.
|
the quantity of matter an object contains
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
5.
|
The diameter of a carbon atom is 0.000 000 000 154 m. What is this number
expressed in scientific notation?
|
|
|
6.
|
The expression of 5008 km in scientific notation is ____.
|
|
|
7.
|
What is the result of multiplying 2.5 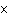 10 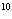 by 3.5 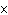
10 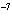 ?
|
|
|
8.
|
What is the result of adding 2.5 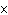 10 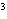 and 3.5 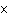
10 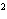 ?
|
|
|
9.
|
The closeness of a measurement to its true value is a measure of its
____.
a. | precision | c. | reproducibility | b. | accuracy | d. | usefulness |
|
|
|
10.
|
Which of the following measurements contains two significant figures?
a. | 0.004 00 L | c. | 0.000 44 L | b. | 0.004 04 L | d. | 0.004 40 L |
|
|
|
11.
|
When a test instrument is calibrated, does its accuracy, precision, or
reliability improve?
a. | precision | c. | reliability | b. | accuracy | d. | all of the
above |
|
|
|
12.
|
Which of the following measurements (of different masses) is the most
accurate?
a. | 3.1000 g | c. | 3.122 22 g | b. | 3.100 00 g | d. | 3.000 000 g |
|
|
|
13.
|
Which group of measurements is the most precise? (Each group of measurements is
for a different object.)
a. | 2 g, 3 g, 4 g | c. | 2 g, 2.5 g, 3 g | b. | 2.0 g, 3.0 g, 4.0 g | d. | 1 g, 3 g, 5 g |
|
|
|
14.
|
Three different people weigh a standard mass of 2.00 g on the same balance. Each
person obtains a reading of 7.32 g for the mass of the standard. These results imply that the balance
that was used is ____.
a. | accurate | c. | accurate and precise | b. | precise | d. | neither accurate nor
precise |
|
|
|
15.
|
Which of the following measurements is expressed to three significant
figures?
a. | 0.007 m | c. | 7.30 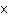 10 10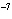 km km | b. | 7077
mg | d. | 0.070
mm |
|
|
|
16.
|
In the measurement 0.503 L, which digit is the estimated digit?
a. | 5 | b. | the 0 immediately to the left of the
3 | c. | 3 | d. | the 0 to the left of the decimal
point |
|
|
|
17.
|
How many significant figures are in the measurement 0.003 4 kg?
a. | two | c. | five | b. | four | d. | This cannot be
determined. |
|
|
|
18.
|
How many significant figures are in the measurement 40,500 mg?
|
|
|
19.
|
How many significant figures are in the measurement 811.40 grams?
|
|
|
20.
|
Express the sum of 1111 km and 222 km using the correct number of significant
digits.
a. | 1300 km | c. | 1333 km | b. | 1330 km | d. | 1333.0 km |
|
|
|
21.
|
Express the sum of 7.68 m and 5.0 m using the correct number of significant
digits.
a. | 12.68 m | c. | 13 m | b. | 12.7 m | d. | 10 m |
|
|
|
22.
|
Express the product of 2.2 mm and 5.00 mm using the correct number of
significant digits.
a. | 10 mm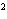 | c. | 11.0 mm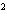 | b. | 11 mm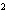 | d. | 11.00
mm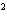 |
|
|
|
23.
|
What is the measurement 111.009 mm rounded off to four significant
digits?
a. | 111 mm | c. | 111.01 mm | b. | 111.0 mm | d. | 110 mm |
|
|
|
24.
|
What is the measurement 1042 L rounded off to two significant digits?
a. | 1.0 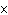 10 10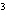 L L | c. | 1050
L | b. | 1040 L | d. | 1.1
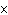 10 10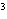 L L |
|
|
|
25.
|
Express the product of 4.0 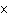 10 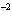 m and 8.1 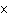
10 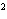 m using the correct number of significant digits.
|
|
|
26.
|
When multiplying and dividing measured quantities, the number of significant
figures in the result should be equal to the number of significant figures in ____.
a. | all of the measurements | b. | the least and most precise
measurements | c. | the most precise measurement | d. | the least precise
measurement |
|
|
|
27.
|
What is the volume of a salt crystal measuring 2.44 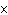 10 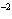 m by 1.4 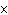 10 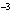 m by 8.4 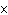
10 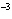 m?
|
|
|
28.
|
What quantity is represented by the metric system prefix deci-?
|
|
|
29.
|
What is the metric system prefix for the quantity 0.000 001?
a. | centi- | c. | kilo- | b. | deci- | d. | micro- |
|
|
|
30.
|
The chief advantage of the metric system over other systems of measurement is
that it ____.
a. | has more units | c. | is in French | b. | is in multiples of 10 | d. | is derived from nature
itself |
|