Matching
|
|
|
Match each item with the correct statement below. a. | mixture | d. | reactant | b. | product | e. | heterogeneous mixture | c. | phase | f. | vapor |
|
|
|
1.
|
gaseous state of substance that is a liquid or solid at room
temperature
|
|
|
2.
|
a physical blend of two or more components
|
|
|
3.
|
part of a sample having uniform composition and properties
|
|
|
4.
|
not uniform in composition
|
|
|
5.
|
a substance formed in a chemical reaction
|
|
|
6.
|
starting substance in a chemical reaction
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
7.
|
Which of the following is NOT an example of matter?
a. | air | c. | smoke | b. | heat | d. | water vapor |
|
|
|
8.
|
A golf ball has more mass than a tennis ball because it ____.
a. | takes up more space | c. | contains different kinds of matter | b. | contains more
matter | d. | has a definite
composition |
|
|
|
9.
|
An example of an extensive property of matter is ____.
a. | temperature | c. | mass | b. | pressure | d. | hardness |
|
|
|
10.
|
All of the following are physical properties of matter EXCEPT ____.
a. | mass | c. | melting point | b. | color | d. | ability to rust |
|
|
|
11.
|
Which of the following is NOT a physical property of water?
a. | It has a boiling point of 100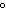 C. C. | b. | It is a colorless
liquid. | c. | It is composed of hydrogen and oxygen. | d. | Sugar dissolves in
it. |
|
|
|
12.
|
Which of the following are considered physical properties of a substance?
a. | color and odor | c. | malleability and hardness | b. | melting and boiling
points | d. | all of the
above |
|
|
|
13.
|
A vapor is which state of matter?
a. | solid | c. | gas | b. | liquid | d. | all of the
above |
|
|
|
14.
|
A substance that forms a vapor is generally in what physical state at room
temperature?
a. | solid | c. | gas | b. | liquid | d. | liquid or solid |
|
|
|
15.
|
Which state of matter has a definite volume and takes the shape of its
container?
a. | solid | c. | gas | b. | liquid | d. | both b and c |
|
|
|
16.
|
Which state of matter takes both the shape and volume of its container?
a. | solid | c. | gas | b. | liquid | d. | both b and c |
|
|
|
17.
|
Which state of matter is characterized by having an indefinite shape, but a
definite volume?
a. | gas | c. | solid | b. | liquid | d. | none of the
above |
|
|
|
18.
|
Which state of matter is characterized by having a definite shape and a definite
volume?
a. | gas | c. | solid | b. | liquid | d. | all of the
above |
|
|
|
19.
|
Which state of matter expands when heated and is easy to compress?
a. | gas | c. | solid | b. | liquid | d. | all of the
above |
|
|
|
20.
|
All of the following are physical properties of a substance in the liquid state
EXCEPT ____.
a. | indefinite volume | c. | not easily compressed | b. | definite
mass | d. | indefinite
shape |
|
|
|
21.
|
Which of the following is a physical change?
a. | corrosion | c. | evaporation | b. | explosion | d. | rotting of food |
|
|
|
22.
|
Which of the following CANNOT be classified as a substance?
a. | table salt | c. | nitrogen | b. | air | d. | gold |
|
|
|
23.
|
Which of the following is a heterogeneous mixture?
a. | air | c. | steel | b. | salt water | d. | soil |
|
|
|
24.
|
Which of the following CANNOT be considered a single phase?
a. | a pure solid | c. | a homogeneous mixture | b. | a pure
liquid | d. | a heterogeneous
mixture |
|
|
|
25.
|
Which of the following is true about homogeneous mixtures?
a. | They are known as solutions. | b. | They consist of two or more
phases. | c. | They have compositions that never vary. | d. | They are always
liquids. |
|