Matching
|
|
|
Match each item with the correct statement below. a. | actual yield | e. | limiting reagent | b. | percent yield | f. | mass | c. | theoretical
yield | g. | number of
molecules | d. | excess reagent | h. | volume |
|
|
|
1.
|
This quantity can always be used in the same way as moles when interpreting
balanced chemical equations.
|
|
|
2.
|
This is conserved only in reactions where the temperature is constant and the
number of moles of gaseous reactants is the same as that of gaseous products.
|
|
|
3.
|
This is conserved in every ordinary chemical reaction.
|
|
|
4.
|
the reactant that determines the amount of product that can be formed in a
reaction
|
|
|
5.
|
the maximum amount of product that could be formed from given amounts of
reactants
|
|
|
6.
|
the reactant that is not completely used up in a reaction
|
|
|
7.
|
the amount of product formed when a reaction is carried out in the
laboratory
|
|
|
8.
|
the ratio of the actual yield to the theoretical yield
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
9.
|
The calculation of quantities in chemical equations is called ____.
a. | stoichiometry | c. | percent composition | b. | dimensional analysis | d. | percent yield |
|
|
|
10.
|
If 1 egg and 1/3 cup of oil are needed for each bag of brownie mix, how many
bags of brownie mix do you need if you want to use up all 3 eggs and 1 cup of oil?
|
|
|
11.
|
What is conserved in the reaction shown below? H 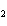 ( g) + Cl 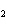 ( g) ®
2HCl( g) a. | mass only | c. | mass, moles, and molecules only | b. | mass and moles
only | d. | mass, moles,
molecules, and volume |
|
|
|
12.
|
What is conserved in the reaction shown below? N 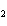 ( g) + 3F 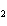 ( g) ®
2NF 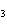 ( g) a. | atoms only | c. | mass and atoms only | b. | mass only | d. | moles only |
|
|
|
13.
|
In every chemical reaction, ____.
a. | mass and molecules are conserved | c. | mass and atoms are
conserved | b. | moles and liters are conserved | d. | moles and molecules are
conserved |
|
|
|
14.
|
In a chemical reaction, the mass of the products ____.
a. | is less than the mass of the reactants | b. | is greater than the mass of the
reactants | c. | is equal to the mass of the reactants | d. | has no relationship to the mass of the
reactants |
|
|
|
15.
|
In any chemical reaction, the quantities that are preserved are ____.
a. | the number of moles and the volumes | b. | the number of molecules and the
volumes | c. | mass and number of atoms | d. | mass and moles |
|
|
|
16.
|
The first step in most stoichiometry problems is to ____.
a. | add the coefficients of the reagents | c. | convert given quantities to
volumes | b. | convert given quantities to moles | d. | convert given quantities to
masses |
|
|
|
17.
|
In the reaction 2CO( g) + O 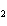 ( g) ® 2CO 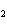 ( g), what is the ratio of moles of oxygen
used to moles of CO 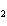 produced?
|
|
|
18.
|
Which of the following is true about the total number of reactants and the total
number of products in the reaction shown below? C 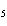 H 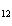 ( l) + 8O 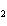 ( g) ®
5CO 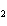 ( g) + 6H 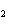 O( g) a. | 9 moles of reactants chemically change into 11 moles of product. | b. | 9 grams of reactants
chemically change into 11 grams of product. | c. | 9 liters of reactants chemically change into 11
liters of product. | d. | 9 atoms of reactants chemically change into 11
atoms of product. |
|
|
|
19.
|
Which of the following is an INCORRECT interpretation of the balanced equation
shown below? 2S( s) + 3O 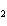 ( g) ®
2SO 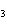 ( g) a. | 2 atoms S + 3 molecules O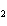 ® 2 molecules SO ® 2 molecules SO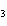 | b. | 2 g S + 3 g
O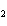 ® 2 g SO ® 2 g SO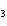 | c. | 2 mol S + 3 mol O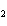 ® 2 mol SO
® 2 mol SO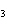 | d. | none of the
above |
|
|
|
20.
|
How many moles of aluminum are needed to react completely with 1.2 mol of
FeO? 2Al( s) + 3FeO( s) ® 3Fe( s) + Al 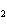 O 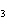 ( s) a. | 1.2 mol | c. | 1.6 mol | b. | 0.8 mol | d. | 2.4 mol |
|
|
|
21.
|
Calculate the number of moles of Al 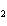 O 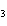 that are produced when 0.60 mol of Fe is produced in the following
reaction. 2Al( s) + 3FeO( s) 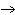 3Fe( s) + Al 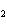 O 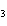 ( s) a. | 0.20 mol | c. | 0.60 mol | b. | 0.40 mol | d. | 0.90 mol |
|
|
|
22.
|
How many moles of glucose, C 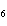 H 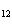 O 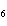 , can be "burned" biologically when
10.0 mol of oxygen is available? C 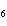 H 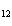 O 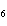 ( s) + 6O 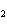 ( g) ®
6CO 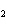 ( g) + 6H 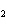 O( l) a. | 0.938 mol | c. | 53.3 mol | b. | 1.67 mol | d. | 60.0 mol |
|
|
|
23.
|
Hydrogen gas can be produced by reacting aluminum with sulfuric acid. How many
moles of sulfuric acid are needed to completely react with 15.0 mol of aluminum? 2Al( s) +
3H 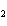 SO 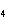 ( aq) ® Al 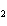 (SO 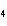 ) 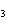 ( aq) + 3H 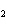 ( g) a. | 0.100 mol | c. | 15.0 mol | b. | 10.0 mol | d. | 22.5 mol |
|
|
|
24.
|
When iron rusts in air, iron(III) oxide is produced. How many moles of oxygen
react with 2.4 mol of iron in the rusting reaction? 4Fe( s) + 3O 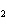 ( g) ® 2Fe2O 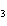 ( s) a. | 1.2 mol | c. | 2.4 mol | b. | 1.8 mol | d. | 3.2 mol |
|
|
|
25.
|
At STP, how many liters of oxygen are required to react completely with 3.6
liters of hydrogen to form water? 2H 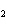 ( g) + O 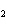 ( g) ® 2H 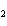 O( g) a. | 1.8 L | c. | 2.0 L | b. | 3.6 L | d. | 2.4 L |
|
|
|
26.
|
Which type of stoichiometric calculation does not require the use of the molar
mass?
a. | mass-mass problems | c. | mass-particle problems | b. | mass-volume
problems | d. | volume-volume
problems |
|
|
|
27.
|
The equation below shows the decomposition of lead nitrate. How many grams of
oxygen are produced when 11.5 g NO 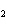 is formed? 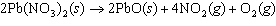 a. | 1.00 g | c. | 2.88 g | b. | 2.00 g | d. | 32.0 g |
|
|
|
28.
|
Iron(III) oxide is formed when iron combines with oxygen in the air. How many
grams of Fe 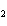 O 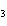 are formed when 16.7 g of Fe
reacts completely with oxygen? 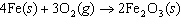 a. | 12.0 g | c. | 47.8 g | b. | 23.9 g | d. | 95.6 g |
|
|
|
29.
|
When glucose is consumed, it reacts with oxygen in the body to produce carbon
dioxide, water, and energy. How many grams of carbon dioxide would be produced if 45 g of C 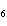 H 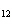 O 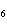 completely reacted with
oxygen? a. | 1.5 g | c. | 11 g | b. | 1.8 g | d. | 66 g |
|
|
|
30.
|
Aluminum reacts with sulfuric acid to produce aluminum sulfate and hydrogen gas.
How many grams of aluminum sulfate would be formed if 250 g H 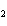 SO 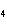 completely reacted with aluminum? 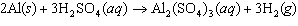 a. | 0.85 g | c. | 450 g | b. | 290 g | d. | 870 g |
|