Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
In a combustion reaction, one of the reactants is ____.
a. | hydrogen | c. | oxygen | b. | nitrogen | d. | a metal |
|
|
|
2.
|
The products of a combustion reaction do NOT include ____.
a. | water | c. | carbon monoxide | b. | carbon dioxide | d. | hydrogen |
|
|
|
3.
|
The type of reaction that takes place when one element reacts with a compound to
form a new compound and a different element is a ____.
a. | combination reaction | c. | single-replacement reaction | b. | decomposition
reaction | d. | double-replacement
reaction |
|
|
|
4.
|
In a double-replacement reaction, the ____.
a. | products are always molecular | b. | reactants are two ionic
compounds | c. | reactants are two elements | d. | products are a new element and a new
compound |
|
|
|
5.
|
Which of the following is a balanced equation representing the decomposition of
lead(IV) oxide?
|
|
|
6.
|
What are the correct formulas and coefficients for the products of the following
double-replacement reaction? RbOH 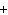 H 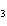 PO 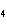
|
|
|
7.
|
Which of the following statements is true about single-replacement
reactions?
a. | They are restricted to metals. | c. | Two reactants produce two
products. | b. | They involve a single product. | d. | Any metal replaces any other
metal. |
|
|
|
8.
|
In the activity series of metals, which metal(s) will displace hydrogen from an
acid?
a. | only metals above hydrogen | c. | any metal | b. | only metals below
hydrogen | d. | only metals from
Li to Na |
|
|
|
9.
|
In the activity series of metals, which metal(s) will not displace hydrogen from
an acid?
a. | only metals above hydrogen | c. | any metal | b. | only metals below
hydrogen | d. | only metals from
Li to Na |
|
|
|
10.
|
Use the activity series of metals to complete a balanced chemical equation for
the following single replacement reaction. Ag( s)  KNO 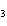 ( aq)
 a. | AgNO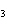 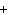 K K | b. | AgK 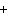 NO
NO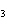 | c. | AgKNO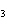 | d. | No reaction takes place because silver is less
reactive than potassium. |
|
|
|
11.
|
Which of the following statements is NOT true about double-replacement
reactions?
a. | The product may precipitate from solution. | b. | The product may be a
gas. | c. | The product may be a molecular compound. | d. | The reactant may be
a solid metal. |
|
|
|
12.
|
In a double-replacement reaction, ____.
a. | the reactants are usually a metal and a nonmetal | b. | one of the reactants
is often water | c. | the reactants are generally two ionic compounds in aqueous
solution | d. | energy in the form of heat or light is often produced |
|
|
|
13.
|
A double-replacement reaction takes place when aqueous Na 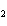 CO 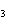 reacts with aqueous Sn(NO 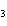 ) 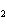 . You would expect one of the products of this
reaction to be ____. a. | NaNO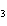 | c. | Sn(CO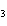 ) )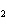 | b. | NaSn | d. | CNO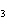 |
|
|
|
14.
|
The complete combustion of which of the following substances produces carbon
dioxide and water?
|
|
|
15.
|
Which of the following is the correctly balanced equation for the incomplete
combustion of heptene, C 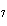 H 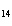 ?
|
|
|
16.
|
The reaction 2Fe 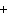 3Cl 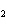 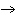 2FeCl 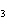 is an example of which type of reaction? a. | combustion reaction | c. | combination reaction | b. | single-replacement reaction | d. | decomposition
reaction |
|
|
|
17.
|
The equation Mg( s) 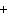 2HCl( aq) 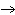 MgCl 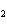 ( aq)
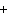 H 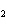 (g) (g) is an example of which type of reaction? a. | combination reaction | c. | decomposition reaction | b. | single-replacement
reaction | d. | double-replacement
reaction |
|
|
|
18.
|
The equation H 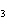 PO 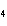 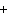 3KOH 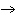 K 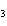 PO 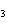 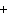 3H 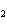 O is an example of which type
of reaction? a. | double-replacement reaction | c. | decomposition
reaction | b. | combination reaction | d. | single-replacement reaction |
|
|
|
19.
|
The equation 2C 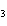 H 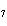 OH 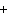 9O 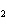  6CO 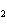 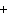
8H 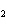 O is an example of which type of
reaction? a. | combustion reaction | c. | double-replacement reaction | b. | single-replacement
reaction | d. | decomposition
reaction |
|
|
|
20.
|
A double-replacement reaction takes place when aqueous cobalt(III) chloride
reacts with aqueous lithium hydroxide. One of the products of this reaction is ____.
a. | Co(OH)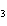 | c. | LiCo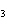 | b. | Co(OH)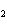 | d. | LiCl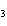 |
|
|
|
21.
|
If a combination reaction takes place between rubidium and bromine, the chemical
formula for the product is ____.
a. | RuBr | c. | RbBr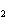 | b. | Rb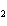 Br Br | d. | RbBr |
|
|
|
22.
|
If a combination reaction takes place between sodium and bromine, the chemical
formula for the product is ____.
a. | SBr | c. | NaBr | b. | Na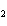 Br Br | d. | NaBr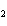 |
|
|
|
23.
|
If a combination reaction takes place between lithium and chlorine, the chemical
formula for the product is ____.
a. | Li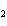 Cl Cl | c. | LiCl | b. | LiCl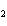 | d. | LCl |
|
|
|
24.
|
If a combination reaction takes place between potassium and bromine, the
chemical formula for the product is ____.
a. | K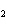 Br Br | c. | PBr | b. | KBr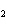 | d. | KBr |
|
|
|
25.
|
If a combination reaction takes place between sodium and iodine, the chemical
formula for the product is ____.
a. | Na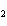 I I | c. | NaI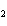 | b. | NaI | d. | SI |
|
|
|
26.
|
If a combination reaction takes place between rubidium and fluorine, the
chemical formula for the product is ____.
a. | RbF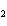 | c. | Rb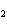 F F | b. | RuF | d. | RbF |
|
|
|
27.
|
If a combination reaction takes place between sodium and fluorine, the chemical
formula for the product is ____.
a. | NaF | c. | SF | b. | NaF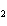 | d. | Na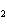 F F |
|
|
|
28.
|
If a combination reaction takes place between rubidium and iodine, the chemical
formula for the product is ____.
a. | RuI | c. | RbI | b. | Rb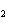 I I | d. | RbI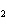 |
|
|
|
29.
|
What is the balanced chemical equation for the reaction that takes place between
bromine and sodium iodide?
|
|
|
30.
|
What is the driving force in the following reaction? Ni(NO 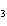 ) 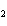 ( aq) 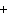 K 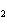 S( aq)  NiS (s) 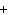 2KNO 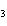 ( aq) a. | A gas is formed. | c. | Ionic compounds are reactants. | b. | A precipitate is
formed. | d. | Ionic compounds are
products. |
|