Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
What is true about the molar mass of chlorine gas?
a. | The molar mass is 35.5 g. | b. | The molar mass is 71.0 g. | c. | The molar mass is
equal to the mass of one mole of chlorine atoms. | d. | none of the
above |
|
|
|
2.
|
What is the number of moles of beryllium atoms in 36 g of Be?
a. | 0.25 mol | c. | 45.0 mol | b. | 4.0 mol | d. | 320 mol |
|
|
|
3.
|
How many moles of CaBr 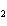 are in 5.0 grams of
CaBr 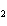 ?
|
|
|
4.
|
What is the mass of silver in 3.4 g AgNO 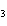 ? a. | 0.025 g | c. | 2.2 g | b. | 0.64 g | d. | 3.0 g |
|
|
|
5.
|
What is the mass of oxygen in 250 g of sulfuric acid, H 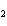 SO 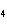 ? a. | 0.65 g | c. | 16 g | b. | 3.9 g | d. | 160 g |
|
|
|
6.
|
The volume of one mole of a substance is 22.4 L at STP for all ____.
a. | gases | c. | solids | b. | liquids | d. | compounds |
|
|
|
7.
|
The molar volume of a gas at STP occupies ____.
a. | 22.4 L | c. | 1 kilopascal | b. | 0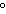 C C | d. | 12
grams |
|
|
|
8.
|
Which combination of temperature and pressure correctly describes standard
temperature and pressure, STP?
a. | 0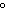 C and 101 kPa C and 101 kPa | c. | 0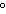 C
and 22.4 kPa C
and 22.4 kPa | b. | 1 C and 0 kPa C and 0 kPa | d. | 100 C
and 100 kPa C
and 100 kPa |
|
|
|
9.
|
The molar mass of a gas can be determined from which of the following?
a. | the density of the gas at STP | c. | Avogadro's
number | b. | the volume of a mole of the gas | d. | none of the
above |
|
|
|
10.
|
What is the volume, in liters, of 0.500 mol of C 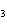 H 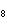 gas at STP? a. | 0.0335 L | c. | 16.8 L | b. | 11.2 L | d. | 22.4 L |
|
|
|
11.
|
What is the number of moles in 500 L of He gas at STP?
a. | 0.05 mol | c. | 22 mol | b. | 0.2 mol | d. | 90 mol |
|
|
|
12.
|
What is the number of moles in 9.63 L of H 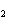 S gas at
STP? a. | 0.104 mol | c. | 3.54 mol | b. | 0.430 mol | d. | 14.7 mol |
|
|
|
13.
|
What is the density at STP of the gas sulfur hexafluoride, SF 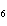 ? a. | 0.153 g/L | c. | 3270 g/L | b. | 6.52 g/L | d. | 3.93 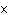 10 10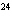 g/L
g/L |
|
|
|
14.
|
The molar mass of a certain gas is 49 g. What is the density of the gas in g/L
at STP?
a. | 3.6 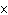 10 10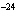 g/L g/L | c. | 2.2
g/L | b. | 0.46 g/L | d. | 71
g/L |
|
|
|
15.
|
A 22.4-L sample of which of the following substances, at STP, would contain 6.02
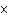 10 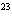 representative particles? a. | oxygen | c. | cesium iodide | b. | gold | d. | sulfur |
|
|
|
16.
|
If the density of an unknown gas Z is 4.50 g/L at STP, what is the molar mass of
gas Z?
a. | 0.201 g/mol | c. | 26.9 g/mol | b. | 5.00 g/mol | d. | 101 g/mol |
|
|
|
17.
|
Which of the following gas samples would have the largest number of
representative particles at STP?
a. | 12.0 L He | c. | 0.10 L Xe | b. | 7.0 L O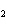 | d. | 0.007 L
SO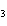 |
|
|
|
18.
|
Given 1.00 mole of each of the following gases at STP, which gas would have the
greatest volume?
a. | He | c. | SO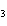 | b. | O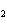 | d. | All would have the same
volume. |
|
|
|
19.
|
If the density of a noble gas is 1.783 g/L at STP, that gas is ____.
|
|
|
20.
|
If 60.2 grams of Hg combines completely with 24.0 grams of Br to form a
compound, what is the percent composition of Hg in the compound?
a. | 28.5% | c. | 71.5% | b. | 39.9% | d. | 60.1% |
|
|
|
21.
|
What is the percent composition of chromium in BaCrO 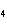 ? a. | 4.87% | c. | 20.5% | b. | 9.47% | d. | 25.2% |
|
|
|
22.
|
If 20.0 grams of Ca combines completely with 16.0 grams of S to form a compound,
what is the percent composition of Ca in the compound?
a. | 1.25% | c. | 44.4% | b. | 20.0% | d. | 55.6% |
|
|
|
23.
|
What is the percent composition of carbon, in heptane, C 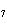 H 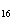 ?
|
|
|
24.
|
What is the percent by mass of carbon in acetone, C 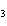 H 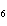 O? a. | 20.7% | c. | 1.61% | b. | 62.1% | d. | 30.0% |
|
|
|
25.
|
Which expression represents the percent by mass of nitrogen in
NH4NO3?
|
|
|
26.
|
Which of the following compounds has the lowest percent gold content by
weight?
a. | AuOH | c. | AuCl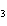 | b. | Au(OH)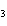 | d. | AuI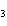 |
|
|
|
27.
|
Which of the following compounds has the highest oxygen content, by
weight?
|
|
|
28.
|
The lowest whole-number ratio of the elements in a compound is called the
____.
a. | empirical formula | c. | binary formula | b. | molecular formula | d. | representative
formula |
|
|
|
29.
|
Which of the following is NOT an empirical formula?
|
|
|
30.
|
Which of the following compounds have the same empirical formula?
|
|
|
31.
|
What is the empirical formula of a compound that is 40% sulfur and 60% oxygen by
weight?
|
|
|
32.
|
What is the empirical formula of a substance that is 53.5% C, 15.5% H, and 31.1%
N by weight?
|
|
|
33.
|
The ratio of carbon atoms to hydrogen atoms to oxygen atoms in a molecule of
dicyclohexyl maleate is 4 to 6 to 1. What is its molecular formula if its molar mass is 280 g?
|
|
|
34.
|
Which of the following is NOT true about empirical and molecular
formulas?
a. | The molecular formula of a compound can be the same as its empirical
formula. | b. | The molecular formula of a compound can be some whole-number multiple of its
empirical formula. | c. | Several compounds can have the same empirical
formula, but have different molecular formulas. | d. | The empirical formula of a compound can be
triple its molecular formula. |
|
|
|
35.
|
Which of the following sets of empirical formula, molar mass, and molecular
formula is correct?
|