Matching
|
|
|
Match each item with the correct statement below. a. | molar volume | b. | molar mass | c. | atomic
mass |
|
|
|
1.
|
the number of grams of an element that is numerically equal to the atomic mass
of the element in amu
|
|
|
2.
|
the mass of a mole of any element or compound
|
|
|
3.
|
the volume occupied by a mole of any gas at STP
|
|
|
Match each item with the correct statement below. a. | representative particle | d. | percent
composition | b. | mole | e. | standard temperature and pressure | c. | Avogadro's number | f. | empirical
formula |
|
|
|
4.
|
the number of representative particles of a substance present in 1 mole of that
substance
|
|
|
5.
|
an atom, an ion, or a molecule, depending upon the way a substance commonly
exists
|
|
|
6.
|
the SI unit used to measure amount of substance
|
|
|
7.
|
0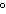 C and 1 atm C and 1 atm
|
|
|
8.
|
the percent by mass of each element in a compound
|
|
|
9.
|
the smallest whole number ratio of the atoms in a compound
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
10.
|
What SI unit is used to measure the number of representative particles in a
substance?
a. | kilogram | c. | kelvin | b. | ampere | d. | mole |
|
|
|
11.
|
How many hydrogen atoms are in 5 molecules of isopropyl alcohol, C 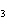 H 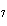 O? a. | 5 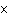 (6.02 (6.02 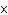 10 10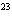 ) ) | c. | 35 | b. | 5 | d. | 35 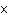 (6.02 (6.02 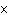 10 10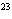 ) ) |
|
|
|
12.
|
Which of the following is NOT a representative particle?
a. | atom | c. | anion | b. | cation | d. | all of the
above |
|
|
|
13.
|
Which of the following elements exists as a diatomic molecule?
a. | neon | c. | nitrogen | b. | lithium | d. | sulfur |
|
|
|
14.
|
Avogadro's number of representative particles is equal to one ____.
a. | kilogram | c. | kelvin | b. | gram | d. | mole |
|
|
|
15.
|
All of the following are equal to Avogadro's number EXCEPT ____.
a. | the number of atoms of bromine in 1 mol Br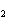 | b. | the number of atoms of gold in 1 mol
Au | c. | the number of molecules of nitrogen in 1 mol N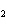 | d. | the number of molecules of carbon monoxide in 1
mol CO |
|
|
|
16.
|
How many moles of tungsten atoms are in 4.8 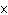 10 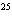 atoms of tungsten?
|
|
|
17.
|
How many moles of silver atoms are in 1.8 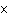 10 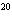 atoms of silver?
|
|
|
18.
|
How many atoms are in 0.075 mol of titanium?
|
|
|
19.
|
How many molecules are in 2.10 mol CO 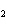 ?
|
|
|
20.
|
How many atoms are in 3.5 moles of arsenic atoms?
|
|
|
21.
|
Butanol is composed of carbon, hydrogen, and oxygen. If 1.0 mol of butanol
contains 6.0 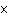 10 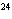 atoms of hydrogen, what is
the subscript for the hydrogen atom in C 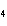 H 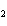 O?
|
|
|
22.
|
The atomic masses of any two elements contain the same number of ____.
a. | atoms | c. | ions | b. | grams | d. | milliliters |
|
|
|
23.
|
Which of the following is NOT a true about atomic mass?
a. | The atomic mass is 12 g for magnesium. | b. | The atomic mass is the mass of one mole of
atoms. | c. | The atomic mass is found by checking the periodic table. | d. | The atomic mass is
the number of grams of an element that is numerically equal to the mass in
amu. |
|
|
|
24.
|
What is true about the molar mass of chlorine gas?
a. | The molar mass is 35.5 g. | b. | The molar mass is 71.0 g. | c. | The molar mass is
equal to the mass of one mole of chlorine atoms. | d. | none of the
above |
|
|
|
25.
|
The mass of a mole of NaCl is the ____.
a. | molar mass | c. | molecular mass | b. | atomic mass | d. | gram atomic
mass |
|
|
|
26.
|
What is the molar mass of AuCl3?
a. | 96 g | c. | 232.5 g | b. | 130 g | d. | 303.6 g |
|
|
|
27.
|
What is the molar mass of (NH 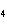 ) 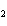 CO 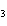 ? a. | 144 g | c. | 96 g | b. | 138 g | d. | 78 g |
|
|
|
28.
|
The molar mass of C 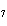 H 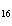
and the molar mass of CaCO 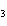 contain approximately the same number of
____. a. | carbon atoms | c. | cations | b. | anions | d. | grams |
|
|
|
29.
|
What is the mass in grams of 5.90 mol C 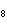 H 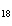 ? a. | 0.0512 g | c. | 389 g | b. | 19.4 g | d. | 673 g |
|
|
|
30.
|
What is the number of moles in 432 g Ba(NO 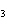 ) 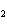 ? a. | 0.237 mol | c. | 1.65 mol | b. | 0.605 mol | d. | 3.66 mol |
|