Matching
|
|
|
Match each item with the correct statement below. a. | molar volume | b. | molar mass | c. | atomic
mass |
|
|
|
1.
|
the number of grams of an element that is numerically equal to the atomic mass
of the element in amu
|
|
|
2.
|
the mass of a mole of any element or compound
|
|
|
3.
|
the volume occupied by a mole of any gas at STP
|
|
|
Match each item with the correct statement below. a. | representative particle | d. | percent
composition | b. | mole | e. | standard temperature and pressure | c. | Avogadro's number | f. | empirical
formula |
|
|
|
4.
|
the number of representative particles of a substance present in 1 mole of that
substance
|
|
|
5.
|
an atom, an ion, or a molecule, depending upon the way a substance commonly
exists
|
|
|
6.
|
the SI unit used to measure amount of substance
|
|
|
7.
|
0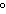 C and 1 atm C and 1 atm
|
|
|
8.
|
the percent by mass of each element in a compound
|
|
|
9.
|
the smallest whole number ratio of the atoms in a compound
|
|
|
Match each item with the correct statement below. a. | product | d. | balanced equation | b. | reactant | e. | skeleton equation | c. | chemical
equation |
|
|
|
10.
|
a chemical equation that does not indicate relative amounts of reactants and
products
|
|
|
11.
|
a new substance formed in a chemical reaction
|
|
|
12.
|
a starting substance in a chemical reaction
|
|
|
13.
|
a concise representation of a chemical reaction
|
|
|
14.
|
an equation in which each side has the same number of atoms of each
element
|
|
|
Match each item with the correct statement below. a. | activity series of metals | c. | combustion
reaction | b. | single-replacement reaction | d. | decomposition reaction |
|
|
|
15.
|
a reaction in which a single compound is broken down into simpler
substances
|
|
|
16.
|
a reaction in which oxygen reacts with another substance, often producing heat
or light
|
|
|
17.
|
a reaction in which the atoms of one element replace the atoms of a second
element in a compound
|
|
|
18.
|
a list of metals in order of decreasing reactivity
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
19.
|
What SI unit is used to measure the number of representative particles in a
substance?
a. | kilogram | c. | kelvin | b. | ampere | d. | mole |
|
|
|
20.
|
How many hydrogen atoms are in 5 molecules of isopropyl alcohol, C 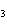 H 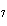 O? a. | 5 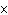 (6.02 (6.02 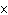 10 10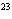 ) ) | c. | 35 | b. | 5 | d. | 35 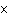 (6.02 (6.02 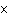 10 10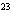 ) ) |
|
|
|
21.
|
Avogadro's number of representative particles is equal to one ____.
a. | kilogram | c. | kelvin | b. | gram | d. | mole |
|
|
|
22.
|
The mass of a mole of NaCl is the ____.
a. | molar mass | c. | molecular mass | b. | atomic mass | d. | gram atomic
mass |
|
|
|
23.
|
What is the molar mass of AuCl3?
a. | 96 g | c. | 232.5 g | b. | 130 g | d. | 303.6 g |
|
|
|
24.
|
What is the molar mass of (NH 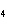 ) 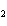 CO 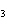 ? a. | 144 g | c. | 96 g | b. | 138 g | d. | 78 g |
|
|
|
25.
|
What does the symbol  in a chemical
equation mean? a. | Heat is supplied to the reaction. | c. | yields | b. | A catalyst is
needed. | d. | precipitate |
|
|
|
26.
|
In the chemical equation H 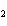 O 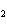 ( aq) ® H 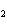 O( l) 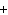
O 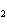 ( g), the 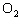 is a ____. a. | catalyst | c. | product | b. | solid | d. | reactant |
|
|
|
27.
|
What are the coefficients that will balance the skeleton equation
below? AlCl 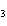 + NaOH 
Al(OH) 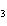 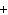 NaCl a. | 1, 3, 1, 3 | c. | 1, 1, 1, 3 | b. | 3, 1, 3, 1 | d. | 1, 3, 3, 1 |
|
|
|
28.
|
What are the coefficients that will balance the skeleton equation below?
N 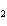 + H 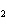 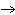 NH 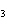 a. | 1, 1, 2 | c. | 3, 1, 2 | b. | 1, 3, 3 | d. | 1, 3, 2 |
|
|
|
29.
|
When the following equation is balanced, what is the coefficient for HCl?
Mg( s) 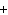 HCl( aq) 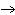
MgCl 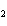 ( aq) 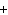 H 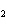 ( g)
|
|
|
30.
|
When potassium hydroxide and barium chloride react, potassium chloride and
barium hydroxide are formed. The balanced equation for this reaction is ____.
|