Matching
|
|
|
Match each item with the correct statement below. a. | halide ion | e. | valence electron | b. | octet rule | f. | coordination number | c. | ionic
bond | g. | metallic
bond | d. | electron dot structure |
|
|
|
1.
|
an electron in the highest occupied energy level of an atom
|
|
|
2.
|
Atoms react so as to acquire the stable electron structure of a noble
gas.
|
|
|
3.
|
a depiction of valence electrons around the symbol of an element
|
|
|
4.
|
an anion of chlorine or other halogen
|
|
|
5.
|
the force of attraction binding oppositely charged ions together
|
|
|
6.
|
the attraction of valence electrons for metal ions
|
|
|
7.
|
the number of ions of opposite charge surrounding each ion in a crystal
|
|
|
Match each item with the correct statement below. a. | ionic bond | d. | coordination number | b. | Lewis electron dot
structure | e. | metallic
bond | c. | valence electron |
|
|
|
8.
|
the attraction of valence electrons for metal
ions
|
|
|
9.
|
an electron in the highest occupied energy level of an
atom
|
|
|
10.
|
the force of attraction binding oppositely charged ions
together
|
|
|
Match each item with the correct statement below. a. | coordinate covalent bond | d. | single covalent
bond | b. | double covalent bond | e. | polar bond | c. | structural formula | f. | hydrogen bond |
|
|
|
11.
|
a depiction of the arrangement of atoms in molecules and polyatomic
ions
|
|
|
12.
|
a covalent bond in which only one pair of electrons is shared
|
|
|
13.
|
a covalent bond in which two pairs of electrons are shared
|
|
|
14.
|
a covalent bond in which the shared electron pair comes from only one of the
atoms
|
|
|
15.
|
a covalent bond between two atoms of significantly different
electronegativities
|
|
|
16.
|
a type of bond that is very important in determining the properties of water
and of important biological molecules such as proteins and DNA
|
|
|
Match each item with the correct statement below. a. | network solid | e. | tetrahedral angle | b. | bonding orbital | f. | VSEPR theory | c. | dipole
interaction | g. | sigma
bond | d. | bond dissociation energy |
|
|
|
17.
|
energy needed to break a single bond between two covalently bonded
atoms
|
|
|
18.
|
symmetrical bond along the axis between the two nuclei
|
|
|
19.
|
molecular orbital that can be occupied by two electrons of a covalent
bond
|
|
|
20.
|
109.5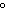
|
|
|
21.
|
shapes adjust so valence-electron pairs are as far apart as possible
|
|
|
22.
|
attraction between polar molecules
|
|
|
23.
|
crystal in which all the atoms are covalently bonded to each other
|
|
|
Match each item with the correct statement below. a. | monatomic ion | f. | cation | b. | acid | g. | binary compound | c. | base | h. | anion | d. | law of definite proportions | i. | polyatomic ion | e. | law of multiple
proportions |
|
|
|
24.
|
atom or group of atoms having a negative charge
|
|
|
25.
|
atom or group of atoms having a positive charge
|
|
|
26.
|
compound composed of two different elements
|
|
|
27.
|
produces a hydrogen ion when dissolved in water
|
|
|
28.
|
produces a hydroxide ion when dissolved in water
|
|
|
29.
|
In any chemical compound, the masses of elements are always in the same
proportion by mass.
|
|
|
30.
|
when two elements form more than one compound, the masses of one element that
combine with the same mass of the other element are in the ratio of small, whole numbers
|