Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
Which
of the following is a balanced chemical equation for the synthesis of NaBr from Na and
Br2? a. | Na +
Br2 ® NaBr | b. | 2Na + Br2 ® NaBr | c. | Na +
Br2 ® 2NaBr | d. | 2Na + Br2 ® 2NaBr | | |
|
|
|
2.
|
Which
of the following is a balanced chemical equation for the synthesis of H2O from
H2 and O2? a. | H2 + O2 ®
H2O | b. | H2 + O2 ®
2H2O | c. | 2H2 + O2 ®
H2O | d. | 2H2 + O2 ®
2H2O | | |
|
Completion
Complete each sentence or
statement.
|
|
|
3.
|
The
statement that in chemical reactions, the total mass of the reactants equals the total mass of the
products is the law of _________________________.
|
|
|
4.
|
In an
experiment, 44 g of propane was burned, producing 132 g of carbon dioxide and 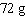 of water. The mass of oxygen that was needed for the reaction was
____________________. of water. The mass of oxygen that was needed for the reaction was
____________________.
|
|
|
5.
|
A(An)
____________________ is the number that appears before a formula in a chemical
equation.
|
|
|
6.
|
The
molar mass of carbon is ____________________.
|
|
|
7.
|
The
molar mass of krypton is ____________________.
|
|
|
8.
|
The
molar mass of chlorine is ____________________.
|
|
|
9.
|
The
molar mass of boron is ____________________.
|
|
|
10.
|
The
molar mass of nitrogen is ____________________.
|
|
|
11.
|
A
sample of NaCl contains 188 g of the compound. The sample contains ____________________ moles of
NaCl.
|
|
|
12.
|
A
sample of NaCl contains 151 g of the compound. The sample contains ____________________ moles of
NaCl.
|
|
|
13.
|
A
sample of HCl contains 183 g of the compound. The sample contains ____________________ moles of
HCl.
|
|
|
14.
|
A
sample of HBr contains 188 g of the compound. The sample contains ____________________ moles of
HBr.
|
|
|
15.
|
A
sample of HCl contains 169 g of the compound. The sample contains ____________________ moles of
HCl.
|
|
|
16.
|
Butane burns as shown in the balanced chemical equation 2C4H10 +
13O2 ® 10H2O + 8CO2. If 6 mol of butane burn,
____________________ mol of carbon dioxide are produced.
|
|
|
17.
|
An
iron fence is left unpainted, and it reacts with the oxygen in the air, forming rust. The formation
of rust is an oxidation-reduction reaction, but it is also an example of a(an) ____________________
reaction.
|
|
|
18.
|
Single-replacement reactions can take place with nonmetals. In the following equation,
assume that A and C are nonmetals and B is a metal. Complete the following general equation for the
replacement of a nonmetal in a compound by another nonmetal: A + BC ®
____________________.
|
|
|
19.
|
The
element ____________________ is always present in a combustion reaction.
|
|
|
20.
|
In a
double-replacement reaction, there are two reactants and ____________________
product(s).
|
|
|
21.
|
When
fluorine reacts with a metal, it forms an F– ion. The fluorine atom has gained an
electron and undergone ____________________.
|
|
|
22.
|
During a chemical reaction, energy is released during the ____________________ of
chemical bonds.
|
|
|
23.
|
In
terms of energy, the general chemical equation AB + CD + energy ® AD + CB
represents a(an) ____________________ reaction.
|
|
|
24.
|
Cooking requires continuous addition of energy to the chemical reactions that are
taking place. The chemical reactions involved in cooking can be described as
____________________.
|
|
|
25.
|
For a
certain chemical reaction, the reactants contain 385 kJ of chemical energy, and the products contain
366 kJ of chemical energy. In order for energy to be conserved, 19 kJ of energy must be
____________________.
|
|
|
26.
|
Measuring how quickly a reactant disappears is one way to measure the
____________________ of the reaction.
|
|
|
27.
|
A
chunk of limestone, which is calcium carbonate, reacts with acid at a certain rate. If the limestone
were crushed, the rate of reaction between the acid and the limestone would
____________________.
|
|
|
28.
|
A
catalyst is used in a catalytic converter in vehicles to help control pollution. For example, the
catalytic converter ____________________ the rate at which carbon monoxide is oxidized to carbon
dioxide. (2CO + O2 ® 2CO2)
|
|
|
29.
|
The
statement that when a change is introduced to a system in equilibrium, the equilibrium shifts in the
direction that relieves the stress on the system is known as _________________________.
|
|
|
30.
|
Many
manufacturing processes involve chemical reactions that reach equilibrium. One way to increase the
amount of product formed is to decrease the ____________________ of the product in the
system.
|