Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
The modern periodic table is arranged in order of increasing atomic ____.
a. | mass | c. | number | b. | charge | d. | radius |
|
|
|
2.
|
Of the elements Pt, V, Li, and Kr, which is a nonmetal?
|
|
|
3.
|
The atomic number of an element is the total number of which particles in the
nucleus?
a. | neutrons | c. | electrons | b. | protons | d. | protons and
electrons |
|
|
|
4.
|
Which subatomic particle plays the greatest part in determining the properties
of an element?
a. | proton | c. | neutron | b. | electron | d. | none of the
above |
|
|
|
5.
|
What is the charge of a cation?
a. | a positive charge | b. | no charge | c. | a negative
charge | d. | The charge depends on the size of the nucleus. |
|
|
|
6.
|
The metals in Groups 1A, 2A, and 3A ____.
a. | gain electrons when they form ions | c. | all have ions with a 1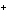 charge charge | b. | all form ions with a negative
charge | d. | lose electrons when
they form ions |
|
|
|
7.
|
How many valence electrons are in an atom of phosphorus?
|
|
|
8.
|
What is the name given to the electrons in the highest occupied energy level of
an atom?
a. | orbital electrons | c. | anions | b. | valence electrons | d. | cations |
|
|
|
9.
|
How does calcium obey the octet rule when reacting to form compounds?
a. | It gains electrons. | b. | It gives up electrons. | c. | It does not change
its number of electrons. | d. | Calcium does not obey the octet
rule. |
|
|
|
10.
|
Which of the following occurs in an ionic bond?
a. | Oppositely charged ions attract. | b. | Two atoms share two
electrons. | c. | Two atoms share more than two electrons. | d. | Like-charged ions
attract. |
|
|
|
11.
|
What is the basis of a metallic bond?
a. | the attraction of metal ions to mobile electrons | b. | the attraction
between neutral metal atoms | c. | the neutralization of protons by
electrons | d. | the attraction of oppositely charged ions |
|
|
|
12.
|
An ionic bond is a bond between ____.
a. | a cation and an anion | c. | the ions of two different metals | b. | valence electrons
and cations | d. | the ions of two
different nonmetals |
|
|
|
13.
|
What type of ions have names ending in -ide?
a. | only cations | c. | only metal ions | b. | only anions | d. | only gaseous
ions |
|
|
|
14.
|
When Group 2A elements form ions, they ____.
a. | lose two protons | c. | lose two electrons | b. | gain two protons | d. | gain two
electrons |
|
|
|
15.
|
What determines that an element is a metal?
a. | the magnitude of its charge | c. | when it is a Group A
element | b. | the molecules that it forms | d. | its position in the periodic table |
|
|
|
16.
|
How are chemical formulas of binary ionic compounds generally written?
a. | cation on left, anion on right | b. | anion on left, cation on
right | c. | Roman numeral first, then anion, then cation | d. | subscripts first,
then ions |
|
|
|
17.
|
Which of the following correctly shows a prefix used in naming binary molecular
compounds with its corresponding number?
a. | deca-, 7 | c. | hexa-, 8 | b. | nona-, 9 | d. | octa-, 4 |
|
|
|
18.
|
When dissolved in water, acids produce ____.
a. | negative ions | c. | hydrogen ions | b. | polyatomic ions | d. | oxide ions |
|
|
|
19.
|
What SI unit is used to measure the number of representative particles in a
substance?
a. | kilogram | c. | kelvin | b. | ampere | d. | mole |
|
|
|
20.
|
How many hydrogen atoms are in 5 molecules of isopropyl alcohol, C 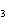 H 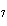 O? a. | 5 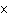 (6.02 (6.02 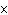 10 10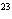 ) ) | c. | 35 | b. | 5 | d. | 35 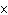 (6.02 (6.02 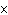 10 10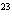 ) ) |
|
|
|
21.
|
Avogadro's number of representative particles is equal to one ____.
a. | kilogram | c. | kelvin | b. | gram | d. | mole |
|
|
|
22.
|
What is the molar mass of AuCl3?
a. | 96 g | c. | 232.5 g | b. | 130 g | d. | 303.6 g |
|
|
|
23.
|
The molar volume of a gas at STP occupies ____.
a. | 22.4 L | c. | 1 kilopascal | b. | 0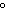 C C | d. | 12
grams |
|
|
|
24.
|
The lowest whole-number ratio of the elements in a compound is called the
____.
a. | empirical formula | c. | binary formula | b. | molecular formula | d. | representative
formula |
|
|
|
25.
|
In the chemical equation H 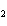 O 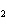 ( aq) ® H 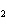 O( l)
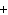 O 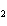 ( g), the 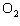 is a ____. a. | catalyst | c. | product | b. | solid | d. | reactant |
|
|
|
26.
|
A catalyst is ____.
a. | the product of a combustion reaction | b. | not used up in a reaction | c. | one of the reactants
in single-replacement reactions | d. | a solid product of a
reaction |
|
|
|
27.
|
What are the coefficients that will balance the skeleton equation
below? AlCl 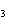 + NaOH  Al(OH) 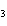  NaCl a. | 1, 3, 1, 3 | c. | 1, 1, 1, 3 | b. | 3, 1, 3, 1 | d. | 1, 3, 3, 1 |
|
|
|
28.
|
Chemical equations must be balanced to satisfy ____.
a. | the law of definite proportions | c. | the law of conservation of
mass | b. | the law of multiple proportions | d. | Avogadro’s principle
|
|
|
|
29.
|
In every balanced chemical equation, each side of the equation has the same
number of ____.
a. | atoms of each element | c. | moles | b. | molecules | d. | coefficients |
|
|
|
30.
|
The type of reaction that takes place when one element reacts with a compound to
form a new compound and a different element is a ____.
a. | combination reaction | c. | single-replacement reaction | b. | decomposition
reaction | d. | double-replacement
reaction |
|